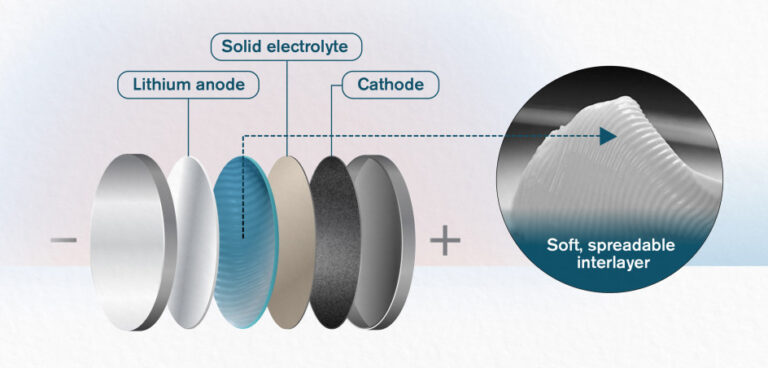
Solid-state batteries are regularly mooted as an alternative to traditional lithium-ion battery technology. In such batteries, liquid electrolyte material, which can be a source of instability in cells, is replaced by a solid medium.
Solid state technology would be particularly attractive in marine applications, where the risk of fire on board vessels is a major consideration (see the latest issue of EHM for details on the Norwegian ferry Ytteroyningen which suffered a battery room fire). Unfortunately, no manufacturer has managed to produce a production-ready solid-state solution that can match the energy and power density of current lithium-ion cells, or provide a long service life.
Many are working on these issues. For example, Samsung recently unveiled a solid-state battery system using a silver-carbon composite layer as an anode, which proved much more stable than lithium (one of the major issues with solid-state lithium batteries is dendrite growth between anode and cathode).
Now, researchers at Chalmers University of Technology, Sweden, and Xi’an Jiaotong University, China, have announced the development of a new interlayer material – which separates the lithium anode, solid electrolyte and whatever cathode material is being used.
Why is such a layer necessary? Simply put, a solid-state battery can be likened to a dry sandwich. A layer of the lithium is the slice of bread, and a ceramic electrolyte is laid on top like a filling. But the ‘sandwich’ is so dry, it is difficult to keep it together – and there are also problems caused by the compatibility between anode/cathode and the electrolyte.
The material researchers in Gothenburg and Xi’an are now working with is a soft, spreadable substance, made of nanoparticles of the ceramic electrolyte LAGP, mixed with an ionic liquid. The liquid encapsulates the LAGP particles and makes the interlayer soft and protective. The material, which its developers say has a similar texture to butter from the fridge, fills several functions and can be spread easily. The main benefit of its use is that it ensures greater stability between the metallic lithium in the cell and the electrolyte – in this case a NASICON (Sodium Super Ionic Conductor) – providing high ionic conductivity but reducing reactivity between the lithium and electrolyte.
The technology has yet to be made production ready, but it is another encouraging step on the road toward solid state batteries, which will benefit both the marine and other hybrid/EV industries.